About Us
Construction
Shimastu batteries are so designed that the necessary quantity of electrolyte is impregnated in the plates and separators to eliminate the need for free electrolyte and to enhance the capability of negative plates to absorb the oxygen by making them moist, thus preventing decrease of the electrolyte and making possible the battery sealed.

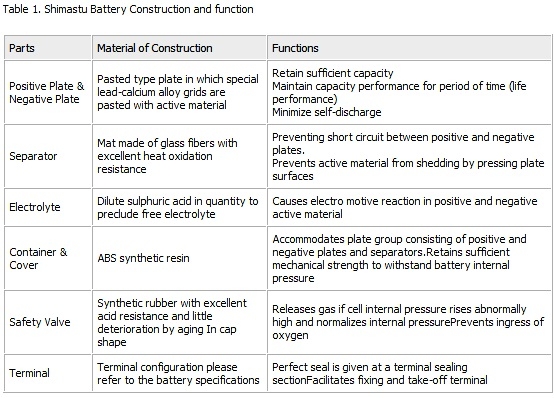
At discharge lead dioxide in positive plates and spongy lead in negative plates react with sulphuric acid in the electrolyte and
gradually transform into lead sulphate, during which the sulphuric acid concentration decreases.
Conversuly, when the battery is charged, the positive and negative active materials which had been turned into lead
sulphate gradually revert to lead dioxide and spongy lead respectively, revert to lead dioxide and spongy lead respectively,
releasing the sulphuric acid absorbed in the active materials, during which the sulphuric acid concentration increases, as shown in Fig.2.
When battery charging approaches its final stage, the charging current is consumed solely for electrolytic decomposition of
water in the electrolyte, resulting in generation of oxygen gas from positive plates and hydrogen gas from negative plates.
The generated gas will escape from the battery causing a decrease of the electrolyte, thereby requiting occasional water replenishment.
However, Shimastu batteries utilize the char-acteristics of spongy lead,or negative active material, which is very active
in moist conditions and reats very quickly with oxygen, thereby suppressing the decrease of water eliminating the need of
water replenishment.
The process of charging from its beginning to the final stage is identical with that of conventional batteries as shown in Fig.2.
Fig.2. Reaction from Beginning of Charge to Before the Final Stage
After the final stage of charging of under overcharge condition, the charging energy is consumed for electrolytic decomposition
of water, and the positive plates generate oxygen gas which reacts with the spongy lead in negative plates and the sulphuric
acid in electrolyte, turning a part of negative plates into a discharged condition, thus suppressing the hydrogen gas generation
from negative plates.
The part of negative plates which had turned to discharged condition through reaction with oxygen gas charging, Thus, a negative
plate keeps equilibrium between the amount which turn into spongy lead by charging and the amount of spongy lead which turns
into lead sulphate through absorbing the gas generated from positive plate, which makes it possible for the battery to be of a
sealed type.
The chemical reaction which takes place after the final stage of charging or under overcharge condition is as shown in Fig.3.,
and the reaction formula is described in the next page.
At discharge lead dioxide in positive plates and spongy lead in negative plates react with sulphuric acid in the electrolyte
and gradually transform into lead sulphate, during which the sulphuric acid concentration decreases.
Conversuly, when the battery is charged, the positive and negative active materials which had been turned into lead sulphate
gradually revert to lead dioxide and spongy lead respectively, revert to lead dioxide and spongy lead respectively, releasing
the sulphuric acid absorbed in the active materials, during which the sulphuric acid concentration increases, as shown in Fig.2.
When battery charging approaches its final stage, the charging current is consumed solely for electrolytic decomposition of
water in the electrolyte, resulting in generation of oxygen gas from positive plates and hydrogen gas from negative plates.
The generated gas will escape from the battery causing a decrease of the electrolyte, thereby requiting occasional water replenishment.
However, Shimastu batteries utilize the char-acteristics of spongy lead,or negative active material, which is very active in
moist conditions and reats very quickly with oxygen, thereby suppressing the decrease of water eliminating the need of water
replenishment.
The process of charging from its beginning to the final stage is identical with that of conventional batteries as shown in Fig.2.
Fig.2. Reaction from Beginning of Charge to Before the Final Stage
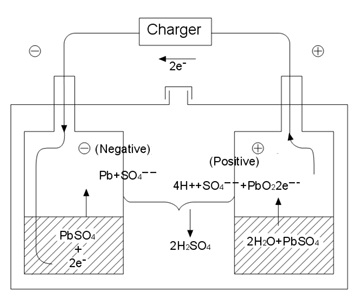
After the final stage of charging of under overcharge condition, the charging energy is consumed for electrolytic decomposition
of water, and the positive plates generate oxygen gas which reacts with the spongy lead in negative plates and the sulphuric
acid in electrolyte, turning a part of negative plates into a discharged condition, thus suppressing the hydrogen gas generation
from negative plates.
The part of negative plates which had turned to discharged condition through reaction with oxygen gas charging, Thus, a negative
plate keeps equilibrium between the amount which turn into spongy lead by charging and the amount of spongy lead which turns into
lead sulphate through absorbing the gas generated from positive plate, which makes it possible for the battery to be of a sealed type.
The chemical reaction which takes place after the final stage of charging or under overcharge condition is as shown in Fig.3.,
and the reaction formula is described in the next page.
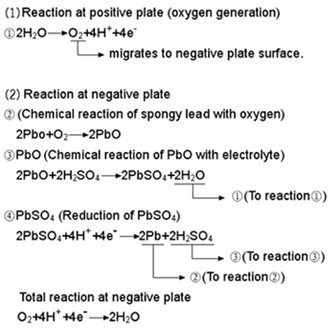
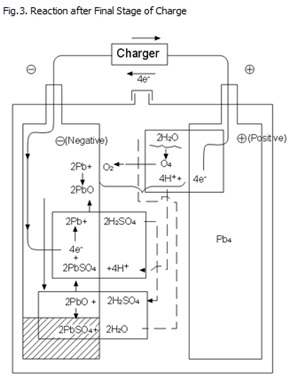
As described above, the oxygen gas generated from the positive plates reacts quickly with the active material in charged condition in the negative plates and returns to water causing very little loss thereof, thus making it possible to build the battery in a sealed construction
Discharge Characteristics
The battery capacity is the parameter to scale the output of the battery discharge. The following factors affect the battery capacity: discharge current, discharge temperature, and final discharge voltage.
Discharge current and discharge capacity
For the same model, the bigger current to discharge, the smaller actual capacity will be.
Fig.4. The relation between discharge rate and discharge time at 25C.
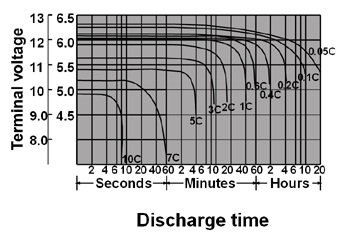
Relationshit of discharge capacity and final discharge voltage
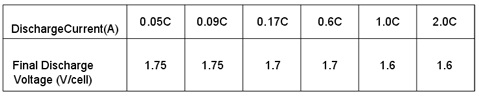
The higher final discharge voltage, the smaller discharge capacity will be; the lower final discharge voltage, the higher
discharge capacity will be. To avoid over-discharge, it is recommended that the lowest final discharge voltage is as following table
Fig.2. Final voltage in different discharge current
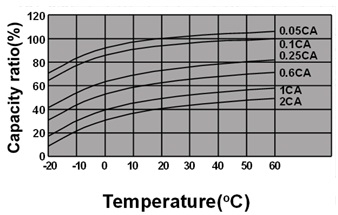
Ambient temperature and discharge capacity
For the same model, in certain range, the higher temperature is, the larger discharge capacity will be.
Fig.5. The relationship between ambient temperature and discharge capacity under different discharge conditions
Self-discharge
When the charged battery is stored or unused, its capacity and voltage will drop gradually, then the battery is in a discharge condition. That is called self-discharge. What should be emphasized is the self-discharge not only proceeds when storing, but also proceeds when charging. Shimastu battery reduces self-discharge by choosing high pure material, and it is only 1/5 of the traditional battery in self-discharge degree.
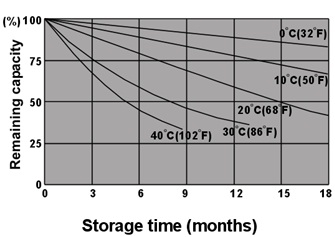
The relation between self-discharge and temperature
The storage temperature influents the self-discharge rate of battery directly, the higher the temperature is, the higher
the self-discharge rate will be.
Fig.6. The relation between remaining capacity and storage time under different temperature
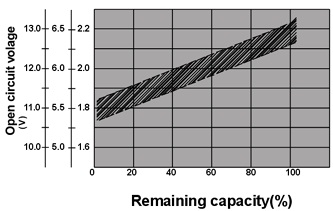
The direct result of self-discharge is the loss of capacity, and it appears as the reducing of open- circuit voltage.
So sometimes the reducing of open- circuit voltage are used to measure the depth of battery self-discharge.
Fig.7. The relation between open- circuit voltage and the remaining capacity
Supplementary Charge
The direct result of self-discharge is the loss of capacity, and it appears as the reducing of open voltageSelf- discharge
results in the loss of the battery capacity, so after the battery is stored for some time or as the open- circuit voltage
falls to the counterpart at which the remaining capacity is 50%(12.4V for a 12V battery, 6.2V for a 6V battery, 2.06V for
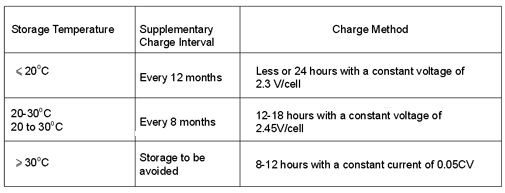
a 2V battery), supplementary charge is very necessary to protect the lead plate from performance losing.
Table 3. Shimastu Battery Supplementary Charge Interval and Charge Method
Constant Power Discharge
Constant Power Discharge means the battery power is constant during discharging. That is the product of discharge current and discharge voltage keeps constant. This discharge method is commonly used for UPS
Charge Characteristics
Regular charge procedure
Charge equipment and charge technology are the main point for charging battery . Generally there are two charging methods:
constant voltage charging and constant current charging. Constant voltage charging is during the charging the voltage is
constant and the charging current drops gradually with the charging depth. Constant current charge is during the charging
the current is constant and the voltage rises gradually with the time. Charging at constant voltage is the most commonly
used method for charging SHIMASTU batteries.
Table 4. Commonly used charging method (25C)
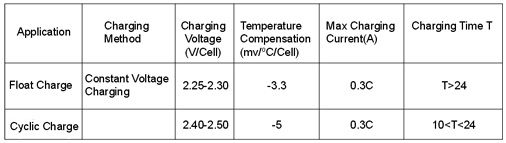
When the temperature deviation is over 10OC, the voltage needs temperature compensation.
For example: Charging a 12V7Ah battery that is for cyclic use, the ambient temperature is 50OC,then the
charging voltage should be designed as: 6X[2.45-(50-25)X0.005]=13.95V.
Charging at over high voltage or charging for over-long time, which will make the water
decomposes then short the battery life. Charge at over low voltage or charging for over
short time, which will cause sulphate gather on lead plate and lead to capacity drop shorting the battery life
Equalized Charge procedure
Shimastu battery is designed as Valve Regulate maintenance free, so the batteries don't need equalized charge. But the changes in environment or in the charging equipment may cause the imbalance inside of the battery series, at this moment it is necessary to carry out equalized charge for a short time to eliminate the imbalance. It is recommended to stop the equalized charge when the voltage is at 2.35-2.38V/cell (25OC) and the battery has bee charging for 48 hours or when the voltage has being at the lowest for 3 hours without rising and the charging current does not drop after continuously charging for 3 hours. Please note the system should be switched to float charge after equalized charge.
Special Charge Procedure
The design of Shimastu Battery allow itself to be over discharged, but serious over discharging will make it fail
to recover capacity even if charging by normal procedure. Therefore, it must be charged by special procedure after
serious over discharge. The charge procedure as follows:
a. Firstly charging the battery at 2.6-3.0V/Cell at least 30 minutes, during the time, the temperature is controlled
and must not be beyond 50OC
b. As the charging current begins to change during the stage of a, please change the voltage into 2.55-2.6V/Cell and
charge the battery.
c. When the charging currents rise and less than 0.1I10A at the stage of b, design the charge voltage as 2.35-2.37V/Cell
and continue to charge for 48-72 hours.
d. At the above every stage, when the temperature rises to 50OC, the charging must be stopped. The charging won't be
carried out until the temperature drop.
After finishing the charging, test the battery by load then confirm the capacity.
Self-life Characteristic
As the battery capacity cannot reach above 50% of the nominal capacity at 20 hours rate, it is called battery life termination.
The factors affect the battery life as following:
Temperature: High temperature will accelerate the battery aging.(Note: charging at very low temperature will tend to generate
hydrogenand shortens battery service life. This is very obvious in float usage. )
Self-discharge depth: The more capacity to be discharged for each time (the more discharge depth), the shorter cycle service
life will be.
The intensity of discharging current: at the same depth of discharge, over big discharging current will shorten the battery
cycle service life.
The intensity of charging current: cycle service life also will be shorted if the battery leaks out gas because of over big
charging current.
Charge and discharge cyclic life
The table shows the relation between cycle numbers and charge depth under some operation condition

Float charge service life
Float service charge life not only determined by the battery itself, but also affected by other factors such as environment temperature, voltage of charge equipment, out put capacity and daily maintenance.
Ambient temperature
When the temperature is above 25OC, it will accelerate to erode the battery plates and shorten the battery life. Valve regulated lead acid battery has many advantages which the traditional one does not have, but it asks for more strict requirement on the operating environment. When using the SLA battery, please pay more attention to the temperature to make sure it is not over 50OC, otherwise the battery may become useless due to out of controlling the hot.
Float charging voltage
Inappropriate float charge voltage will affect battery life and capacity directly, if the float voltage is over high, float current will rise accordingly. That will accelerate to erode the grid, and shorten the battery life. The environment temperature of battery must be considered when confirm the float charge voltage. When there are 10OC difference between temperature and standard environment temperature we need to compensate the float voltage, please refer to Fig 4 to have a general idea of how to adjust the float voltage. If the float voltage are too low, the float current will decrease accordingly ,may lower battery capacity,and the plate sulfated, then shorten the battery life
Parallel connection and connection in series of the battery
Connecting the same specification battery in series or in parallel can get the relevant battery group. But connecting different specification batteries (or battery manufactured by different manufacturer) in series or in parallel is prohibited. For Shimastu battery, to guarantee the reliable operation of the battery group, try to decrease the numbers of group in parallel. If there are too many battery groups in parallel, please choose the batteries with higher capacity.


